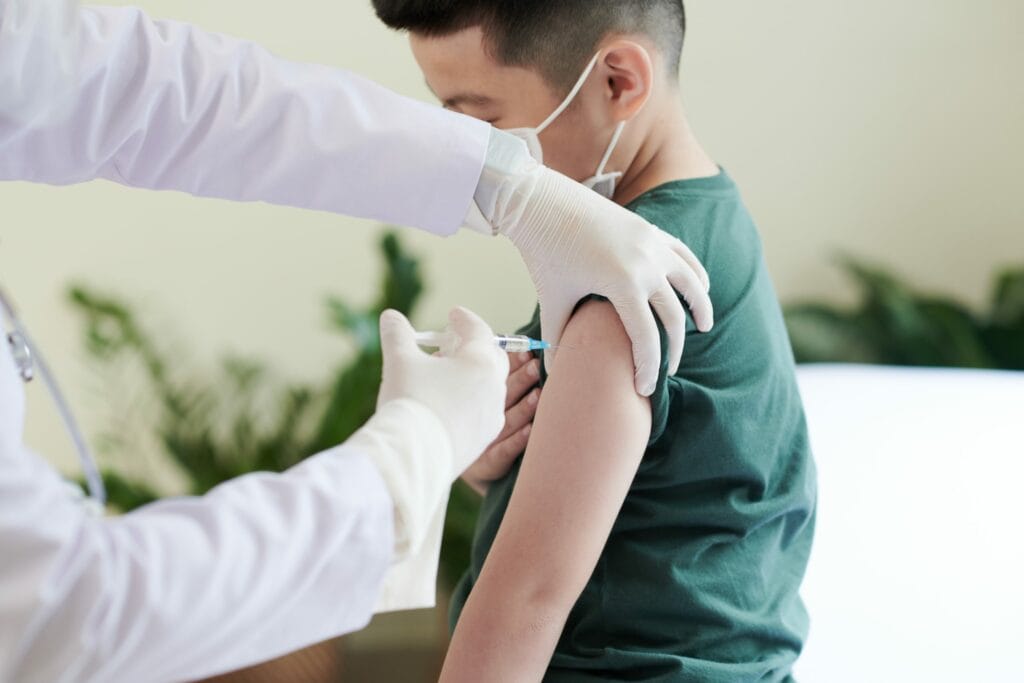
With the Omicron variant of the Coronavirus surging around the world, the FDA has expanded the Pfizer booster shot to be accessible to children as young as 12 years old Monday.
The FDA also said any one aged 12 or older that is eligible for a booster shot can get one in 5 months, shortening the time period of six months.
However, that is not the final bridge to cross as kids are heading back to school.
The Center for Disease Control and Prevention will have to decide to recommend booster shots for kids in their younger teens. Dr. Rochelle Walensky, the CDC’s director, is expected to make that decision later this week.
When it comes to younger children, those aged 5-11 years old, kid sized doses rolled out in November and experts say that healthy children in that age frame should be protected after that second dose for a long amount of time. However, the FDA also said Monday that if children had severely weak immune systems, they will be allowed a third dose 28 days after their second. That is the same time frame for children and adults after getting their second dose and about to receive their third.
Pfizer is currently studying their vaccine as they are looking to make it available in even smaller doses for children aged less than 5 years old.